Understanding the Chemical Structures of Polydopamine
Student: Deborah Takyibea Okyere
Degree: M.S., July 2021
Major Professor: Dr. Jingyi Chen
Research Area(s):
Biological Materials and Processes, Photonics
Background/Relevance
- Polydopamine(PDA) is an analogy of eumelanin with strong adhesion properties and compatibility to biological system with little cytotoxicity. Examples of PDA application include surface coating and platform for drug delivery. However, there are only a few studies on understanding the complex structures of PDA. In order to understand the PDA functionality, its chemical structure needs to be well characterized.
Innovation
- Well–controlled chemical reaction approaches for the synthesis and degradation of PDA are used together with spectroscopic tools to understand the chemical structures of PDA.
Approach
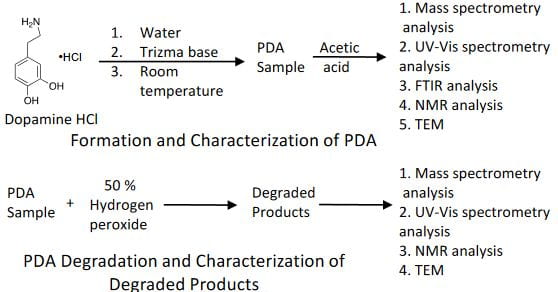
Key Results

Conclusions
- The concentration and molar mass of the oligomers increase with time of formation and their structures can be analyzed using different spectroscopic tools such as mass spectrometry, NMR and FTIR.
- PDA structures are covalently bonded together to form different degree of oligomers. These oligomers are made up of building blocks which consist of dopamine, 5,6–dihydroxyindole and indole–5,6–quinone.
- PDA can be degraded with hydrogen peroxide to form trimer and tetramer units of PDA structures which are water soluble.
