Localized Electrodeposition of Gold Using Photodoped Cuprous Oxide
Student: Elizabeth Johnson
Major Professor: Dr. Robert Coridan
Research Area(s):
Energy Materials & Devices
Background/Relevance
- Traditional lithography of gold is extremely expensive and requires the use of a clean room
- Cuprous oxide is earth abundant and efficient at performing galvanic replacement reactions
- Can locally photoelectrodeposit cuprous oxide using LED light with a photomask, producing photodoped cuprous oxide containing cuprous oxide and copper metal nanocrystals
Innovation
- Can electrodeposit gold onto localized photodoped cuprous oxide, providing a way to produce microprocessors on the bench without the use of a clean room
Approach
- Synthesize gold metal solution
- Using a potentiostat, deposit layer of gold onto FTO, cuprous oxide, and photodoped cuprous oxide to test abilities of gold solution
- Test similar conditions with external illumination (660 nm red LED light)
- Pattern the electrodeposition of gold using a photomask

Key Results
- Before gold starts electroplating onto the substrate, the cuprous oxide first reduces to copper
- The gold layer adheres better to the photodoped cuprous oxide than ordinary cuprous oxide
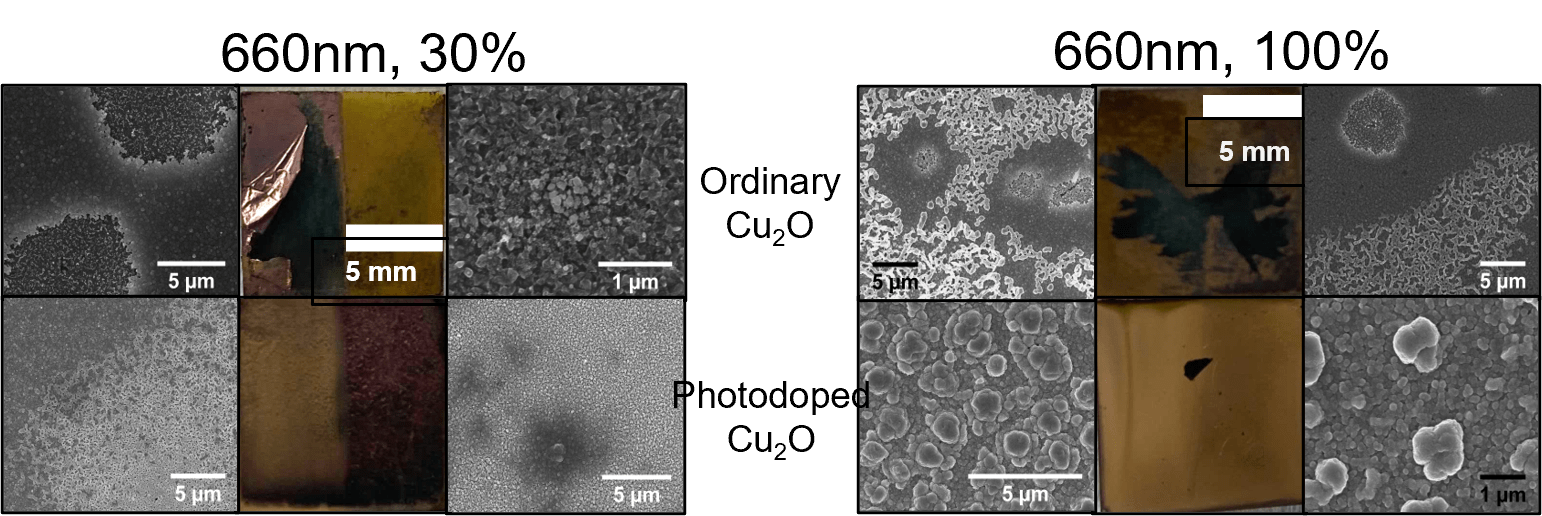
Conclusions
- Adding light to electrodeposition increase the rate of gold electroplating
- There is some heat transfer that occurs between light and dark sides, causing similar electrodeposition rates between lit and non-lit sides of the substrate
- Electrodepositing gold onto localized photodoped cuprous oxide will provide a way to produce inexpensive, noble-metal electrodes for micron-scale processes on the bench without the use of a clean room
