Characterization of FeNi catalyst through TPR and Pulse Chemisorption
Student: Jahmelia Atkinson
Major Professor: Dr. Lauren Greenlee
Research Area(s):
Microelectronics
Nanoscience & Engineering
Background/Relevance
- NH3 is one of the most abundantly produced chemical in the world. It is more famously used in agriculture as a fertilizer.
- It is made under the Haber Bosch process which requires high temperatures (250-300C) and pressures (100-200 atm), it takes up approximately 2% of worlds energy consumption.
- Electrochemical reduction of N2 and H2 has been identified as an alternative approach to produce NH3 at lower conditions.
Innovation
- Design an electrochemical cell to put in the Chemstar for nitrogen reduction reaction.
Approach
- Characterize nanoparticle catalyst through Temperature Programmed reduction and Pulse Chemisorption.
- Synthesize different molar ratio (3:1, 1:1, 6:1) FeNi nanoparticles.
Key Results
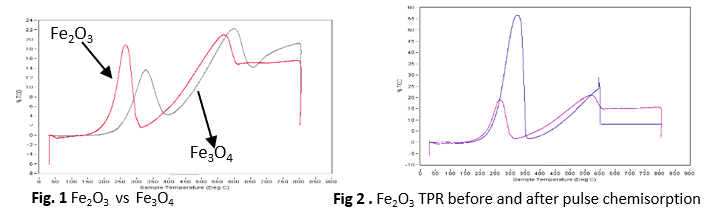
- A comparison of TPR and pulse chemisorption was done on Fe3O4 and Fe2O3 .
- The red curve (Fe2O3 ) shows that pulse chemisorption was done before tpr while the black curve (Fe3O4) shows that pulse chemisorption was done before TPR.
- Figure 2 is a comparison of Fe2O3 Pulse chemisorption before tpr vs pulse chemisorption after TPR.
Conclusions
- It was observed that when pulse chemisorption is done before TPR, a much higher peak is produced.
- Fe3O4 has a higher peak than Fe2O3 which means more gas is been adsorbed when pulse chemisorption is carried out before TPR.
- Multiple peaks indicate the presence of metal in different forms on the support of having different level of between species and support.
- Fe2O3 was reduced by 251.520%
- Fe3O4 was reduced by 497.025%
