Potential Use of Lysenin Channels in Drug Release from Liposomes
Student: Jess Ray
Major Professor: Dr. Gregory Salamo
Research Area(s):
Nanoscience and Engineering
Background/Relevance
- Wild type lysenin channels were investigated under the influence of different multivalent ions.
- Lysenin channels inserted into liposomes could create new opportunities for drug delivery systems.
Innovation
- Use the mutated lysenin channels in experiments to determine the voltage at which they gate and compare it to wild type lysenin.
- Control the drug release out of the lysenin-liposome drug carriers to enhance drug delivery applications.
Approach
- Use Teflon chambers to create a bilayer lipid membrane (BLM) model.
- Create lipid solution containing cholesterol, sphingomyelin, and asolectin to create a model cell like animal cell membranes.
- Insert lysenin in the BLM to experimentally determine gating voltages (using Axo-Patch).
- Introduce multivalent ions to observe the ligand – induced gating.
Key Results
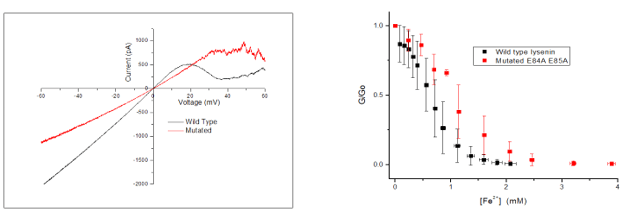
- Wild type lysenin channels gates at roughly 20mV, while the mutated gated at roughly 30-35mV (as the figure on the left shows).
- Iron divalent cations inhibit the macroscopic current of both wild and mutated lysenin channels at low monovalent ions concentration (as the figure on the right shows).
Conclusions
- Mutated type lysenin gated at a higher voltage than the wild type lysenin did, due to the mutation. This lead to roughly a 10-15 mV higher voltage required to cause gating in the mutated type.
- Using a less concentrated monovalent solution (50mM KCl instead of 150mM) allowed the conductance of both types of lysenin to reach zero.
- The charge of the mutated lysenin was reduced by the mutation. This lead to a higher concentration of iron divalent ions being needed for ligand-induced gating, as hypothesized.
