Characterization of Protein Aggregation by Solid State Nanopore
Student: Mitu Acharjee
Degree: Ph.D., May 2021
Major Professor: Dr. Jiali Li
Research Area(s):
Nanoscale Materials & Devices
Biological Materials & Devices
Background/Relevance
-
Many neurodegenerative diseases like Alzheimer, Parkinson and Prion are found to be linked to protein aggregation. Existing protein characterization methods are:
- Not easily available.
- Expensive.
- Low precision rate.

Innovation
- Build a new system that can be used to:
1- Characterize protein aggregation.
2- Low cost and high precision.
3- Easily moveable/reduced size.
Approach
- Proteins translocate through a single nanopore in a Si3N4 membrane that separates two salt solution-filled chambers whose only connection was via the electrolyte (KCl) solution inside the nanopore.
- Protein molecules translocating through a nanopore partially block the ion flow and the current blockage pulse or event can be measured.
-
By measuring the current and using nanopore geometry the translocated protein and its aggregations can be characterized.
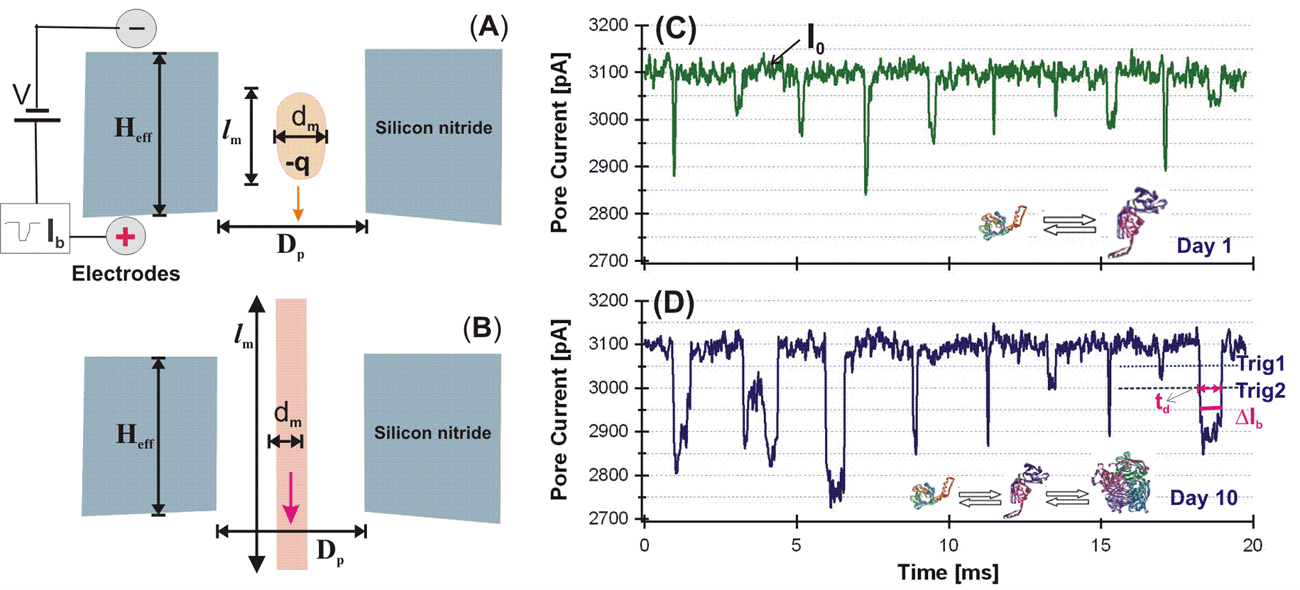
Key Results
- Solid-state nanopore (6-30 nm) has been fabricated and imaged using TEM.
- Tetrameric and hexameric aggregations of β-lactoglobulin protein were detected using 18 nm nanopore.
- Dimeric aggregations of tau protein were detected using 10 nm nanopore as a function of salt concentration and pH.
- The dimeric aggregations of α and β tubulins were detected in 1M KCl solution at 60 – 210 mV.
- The pentameric to heptameric aggregations of tau and tubulin were detected in 1M KCl solution at 60 – 210 mV.
Conclusions
- This work supports the understanding of the theory and principle of:
- Tau and tubulin aggregations in ionic solution using solid-state nanopore device.
- In vitro tau and tubulin aggregations manipulations using pH and salt concentration changing.
- In vitro protein aggregation reduction using applied voltage.
Future Work
-
Study on the mechanisms of protein aggregations in live cells and pH, voltage, temperature and salt effects on the aggregation.
