Deposition of Ni-Fe Core-Shell Nanoparticles on Various Substrates for
the Oxygen Evolution Reaction
Student: Sarah York
Major Professor: Dr. Jingyi Chen
Research Area(s):
Nanoscale Materials & Devices
Energy Materials and Devices
Background/Relevance
- Due to the lethargic kinetics of the oxygen evolution reaction in comparison to the hydrogen evolution reaction portion of water splitting, there has been a search for different catalysts that can be mass produced with a reasonable price and activity.
- Industrialization of a catalyst would allow for more efficient production, and thus storage, of hydrogen for fuel cells.
Innovation
- Deposition of the Ni-Fe on various nickel foams to attempt to increase the catalytic activity for the oxygen evolution reaction.
- Optimization of the process for depositing nanoparticles.
Approach
-
- Nickel nanoparticle cores were synthesized then coated with iron.
- A diluted solution of the nanoparticle samples were imaged using TEM to determine the morphology of the nanoparticles.
- Ni-Fe nanoparticles of higher catalytic activity with varying ligands were deposited onto the selected substrates.
- Ink prep testing was performed on carbon paper before activity testing of the catalyst on the substrates.
Key Results
Conclusions
-
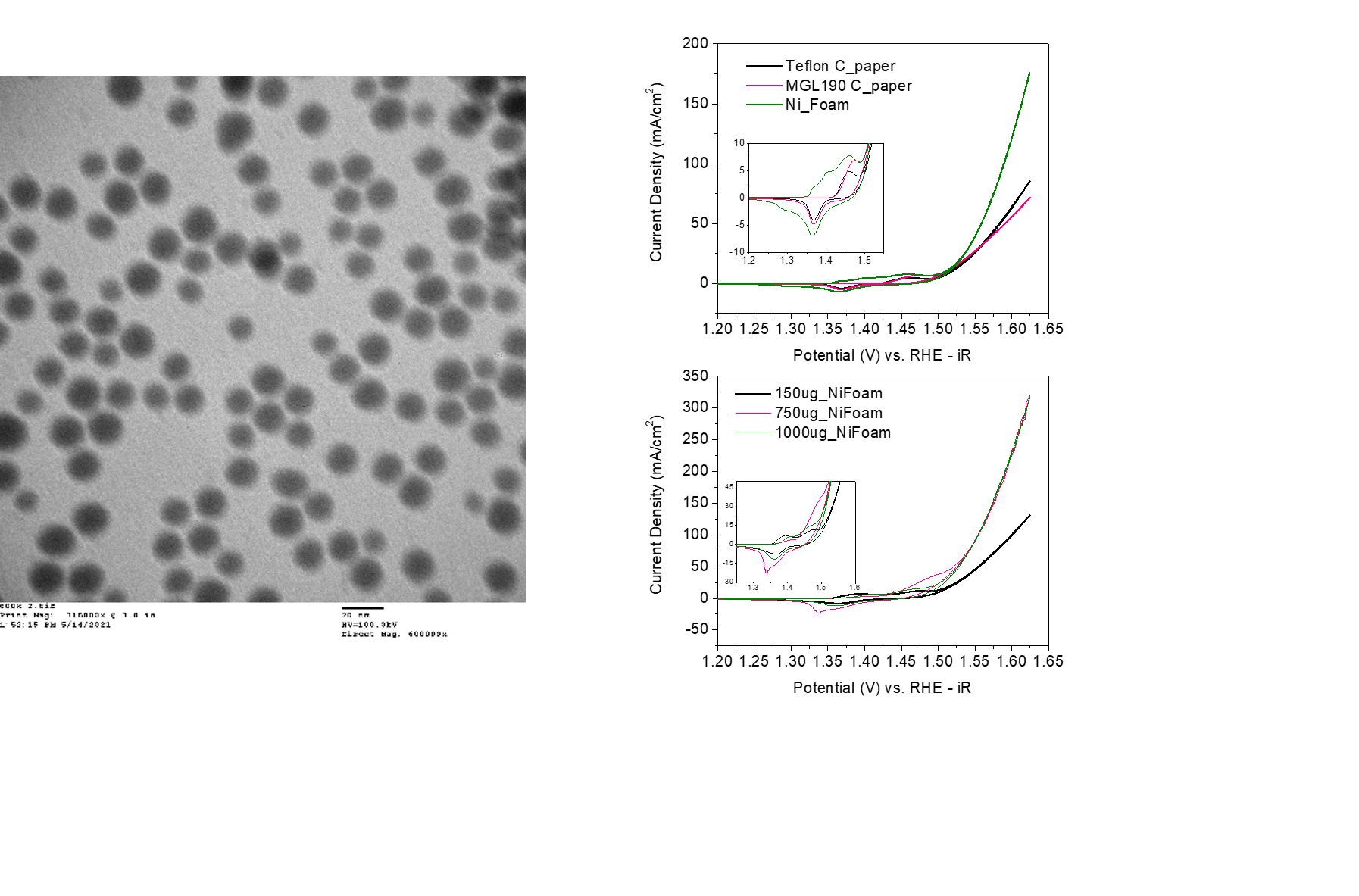
The TEM images confirmed the nanoparticles to be of a generally consistent size at about 13 nm.
-
The best ink prep was determined to be the entirely ethanol ink prep from the carbon paper testing comparisons on cyclic voltammetry (CV) graphs.
-
Nickel foam is the best substrate due to the nature of its relatively high capacitance and low resistance and high activity as seen on the 30th CV graphs.
-
The loading test of different increased concentrations on the nickel foam showed a plateau at 1000 mg loading.
