Electrochemical Deposition in Energy Storage Devices
Student: Witness Martin
Degree: Ph.D, July 2021
Major Professor: Dr. Jie Xiao
Research Area(s):
Conventional Materials and Processes
Nanoscience & Engineering
Background/Relevance
-
- Conventional energy storage devices utilize either aqueous or non-aqueous electrolytes to facilitate the electrochemical deposition of metals within the liquid electrolyte.
- In the presence of organic solvents, a passivating solid electrolyte interface (SEI) develops on the surface of metals such as lithium during the electrochemical deposition process. While in aqueous systems this passivation layer does not exist.
- By studying the deposition processes of metal ions in the presence and absence of the SEI a deeper understanding of the factors influencing the cycling of Li metal can be garnered to develop next generation Li metal batteries.
Approach
- Investigate the diffusion, nucleation and growth of silver under varying currents, various anions, and cation concentrations in an aqueous system.
- Develop novel electrolytes for use in Li-S battery systems through carefully selected solvents, co-solvents and additives.
- Investigate the diffusion, nucleation and growth of Li under varying deposition currents, various anions, Li solvation structures and cation concentrations in the non-aqueous system using a microelectrode tool.
- Investigate novel electrolytes in a Li-S coin cell setup.
Key Results
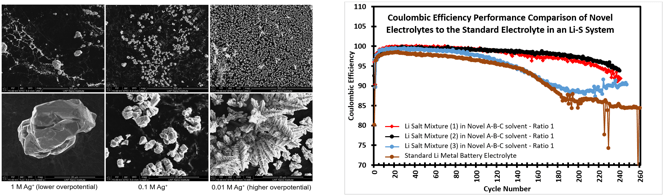
- An increase of the Ag cation concentration resulted in a reduced nucleation density as shown in the SEM imaging below.
- Deposition current density also has an affect on the nucleation rate and crystal size of the deposited Ag material.
- The novel non-aqueous electrolytes resulted in an improvement of the Coulombic Efficiency of the Li-S coin cell.
Conclusions
-
Cation concentration adjustment has a significant affect on concentration gradient of the system which has a resultant affect on the nucleation and morphology of the deposited material.
-
Increased current density results in faster system kinetics and a propensity towards dendritic growth of the metal.
-
Key performance indicators have shown that the novel non-aqueous electrolytes offer a potential avenue to improve the Li-S system.
Future Work
-
Expand the study of the novel non-aqueous electrolytes to observe their performance in a Li-S system.
