Investigating the Electrochemical Behavior of Silver Nanoparticles at Short Time Scales
Student: Moriah York
Major Professor: Dr. Ingrid Fritsch
Research Area(s):
Nanoscience & Engineering
Background/Relevance
- Surge of consumer products containing silver nanoparticles (AgNP).
- Necessity to develop methods that monitor real time changes of AgNP in different solutions.
Innovation
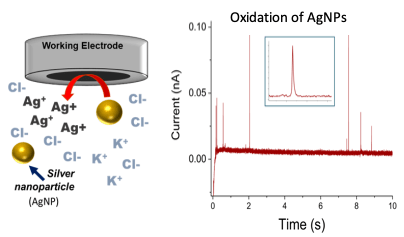
- Study the effect of electrolyte concentration on the frequency of electrochemical reactions between AgNP and electrode.
Approach
- Polish electrode and characterization with cyclic voltammetry to ensure absence of AgNPs.
- Prepare electrolyte and AgNP solution and allow equilibration (soak) time before inserting electrodes or not (no soak).
- Perform four sets of ten (10 s) CAs at varying potentials to observe the occurrence of particle oxidations at the electrode.
- Analyze the frequency of the particle oxidation peaks and the effects of the soaking time and varying electrolyte concentrations and equilibration time.
- Confirm above studies by performing additional studies with various electrolyte and particle concentrations.
Key Results
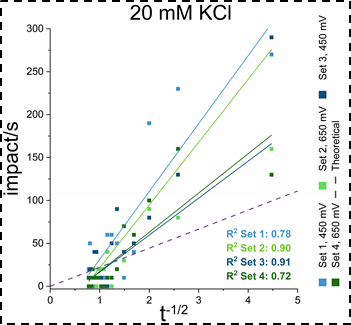
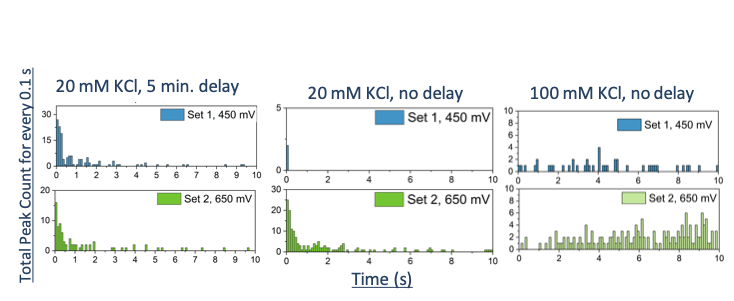
- For lower electrolyte concentrations, the oxidation frequency was greater for the first few seconds and for higher electrolyte concentration, the oxidation frequency was overall steady.
- Equilibration period of AgNP in electrolyte solution was needed before oxidation could be observed.
Conclusions
- The results of the experiments attempted to fit a model, the Cottrell equation to the initial oxidation frequency behaviour.
- Further studies are required to vary electrolyte and particle concentrations and to fit a model that accurately accounts for the electrolyte and kinetics of the system.